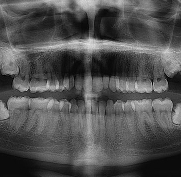
Study status – The service evaluation aims to assess the current practice in relation to lower wisdom teeth and data collected may be used to inform future ethically approved research.
To read more about this study, visit the NFORC website.
The project is funded by Saving Faces – The Facial Surgery Research Foundation and co-ordinated by the National Facial and Oral Research Centre (NFORC), a branch of Saving Faces.
Consultant in Oral and Maxillofacial Surgery & Sub Specialist Interest Group Lead for Dento-Alveolar & Oral Surgery – BAOMS
Mr Geoff Chiu
Professor of Oral and Maxillofacial Surgery & Honorary Consultant in Oral Surgery with Barts Health NHS Trust – BAOS
Prof Paul Coulthard
NFORC Director
Prof Iain Hutchison
Clinical Research Manager
Fran Ridout